AMMF’s Virtual 2021 European Cholangiocarcinoma Conference
Abstract No 11
Efficacy and safety of pemigatinib in European patients with previously treated locally advanced or metastatic cholangiocarcinoma: a FIGHT-202 subgroup analysis
Background and Aims
Pemigatinib, a potent and selective oral fibroblast growth factor receptor (FGFR) 1-3 inhibitor is approved in the US, Europe, and Japan for previously treated advanced CCA harboring FGFR2 fusion/rearrangements based on results from FIGHT-202 (NCT02924376; objective response rate [ORR], 35.5%; disease control rate [DCR], 82%; median duration of response [mDOR], 7.5 months; progression-free survival [mPFS], 6.9 months; overall survival (mOS, not mature), 21.1 months) [Abou-Alfa. Lancet Oncol. 2020;2:671-684]. We conducted a post-hoc subgroup analysis of European patients enrolled in FIGHT-202.
Methods
Patients with advanced disease and progression after ≥1 prior treatment were assigned based on confirmed FGF/FGFR status to cohort A (FGFR2 rearrangement), B (other FGF/FGFR genetic alterations [GAs]), or C (no FGF/FGFR GAs). Patients received pemigatinib 13.5 mg QD (21-day cycle; 2-weeks-on, 1-week-off) until disease progression/unacceptable toxicity. Primary endpoint: centrally confirmed ORR in cohort A.
Results
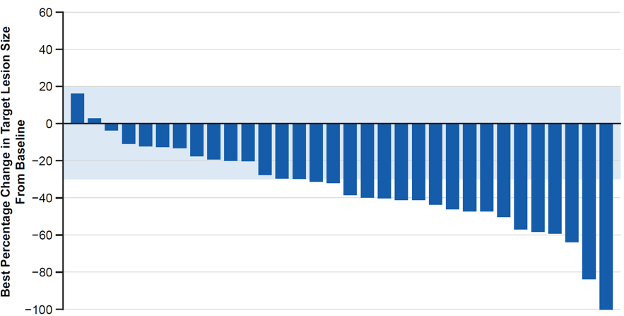
Of 147 patients enrolled in FIGHT-202 at data cutoff (Mar 22, 2019), 35 (24%) were from Europe (cohort A, n=32; B, n=3; C=0; France, n=10; Italy, n=8; other country, n=17; median (range) age, 59 (34-77) years; ≥2 prior therapies, 46%). Of the 35 patients, 25 discontinued treatment (disease progression, 84%); 10 continued treatment (all cohort A). In cohort A, ORR (95% CI) was 40.6% (23.7-59.4) with 1 complete response (Figure); mDOR was 7.5 months (5.5-7.5), DCR was 87.5% (71.0-96.5), mPFS was 6.9 months (4.8-9.1), and mOS was 14.7 months (11.7-not reached). In cohort B, 2 patients had stable disease (mPFS, 2.1 and 4.0 months). Common AEs were diarrhoea (80%), alopecia (66%), and hyperphosphataemia (60%); 14%, 60% and 3% of patients had hypophosphataemia, nail toxicities, and retinal detachment, respectively. AEs led to dose reductions and interruptions in 20% and 57% of patients.
Conclusions
Efficacy and safety of pemigatinib in European patients were similar to published findings in the total study population.
Figure. Best percentage change in target lesion size in European patients enrolled in FIGHT-202 (cohort A)
Authors
1.Antoine Hollebecque (HOLLEBECQUE@gustaveroussy.fr), France
2.Davide Melisi (melisi@univr.it), Italy
3.Arndt Vogel (vogela@me.com), Germany
4.Mairéad Geraldine McNamara (mcnamara@nhs.net), UK
5.Teresa Macarulla (tmacarulla@vhio.net), Spain
6.Eric Van Cutsem (vancutsem@uzleuven.be), Belgium
7.Huiling Zhen (hzhen@incyte.com), USA
8.Luis Féliz (LFeliz@incyte.com), Switzerland
9.Ghassan K. Abou-Alfa (abou-alg@MSKCC.ORG), USA
Affiliations
1.Department of Adult Medicine, Gustave Roussy, Villejuif, France
2.Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy
3.Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Niedersachsen, Germany
4.Division of Cancer Sciences, University of Manchester, and Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester, UK
5.Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology, Barcelona, Spain
6.Department of Digestive Oncology, University Hospitals Leuven and KU Leuven, Leuven, Belgium
7.Incyte Corporation, Wilmington, DE, USA
8.Incyte Biosciences International Sàrl, Morges, Switzerland
9.Department of Medicine, Memorial Sloan Kettering Cancer Center and Weill Medical College at Cornell University, New York, NY, USA
Disclosures
Antoine Hollebecque:
Advisory/Consultancy – Amgen, AstraZeneca, Debiopharm, Incyte Corporation, QED Therapeutics, Lilly
Davide Melisi:
Research funding – Celgene, Evotec, Incyte Corporation, Shire; consulting role: Baxter, Eli Lilly, Evotec, Incyte Corporation, Shire
Arndt Vogel:
Advisory/consultancy – AstraZeneca, Bayer, Bristol-Myers Squibb, BTG, Eisai, Eli Lilly and Company, Incyte Corporation, Ipsen, Janssen, Merck, MSD, Novartis, Pierre Fabre, Roche, Sanofi, and Servier; honoraria – AstraZeneca, Bayer, Bristol-Myers Squibb, BTG, Eisai, Eli Lilly and Company, Incyte Corporation, Ipsen, Janssen, Merck, MSD, Novartis, Pierre Fabre, Roche, Sanofi, and Servier
Mairéad Geraldine McNamara:
Honoraria – Ipsen, NuCana and Mylan; research funding – Ipsen, NuCana and Servier (previously SHIRE); travel assistance – Ipsen, Bayer and Novartis.
Teresa Macarulla:
Research support – AstraZeneca, Bei-Gene, Celgene; speakers’ bureau honoraria – Celgene, Incyte Corporation, Raffo, Sanofi, Servier; consultant/advisory board member – Amgen, AstraZeneca, Celgene, Incyte Corporation, Servier.
Eric Van Cutsem:
Advisory/consultancy – Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Incyte Corporation, Lilly, Merck KGaA, Merck Sharp & Dohme, Novartis, Roche, Servier; research grant/funding – Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Ipsen, Lilly, Merck KGaA, Merck Sharp & Dohme, Novartis, Roche, Servier
Huiling Zhen:
Employment and stock ownership – Incyte Corporation
Luis Feliz:
Employment and stock ownership – Incyte Corporation
Ghassan K. Abou-Alfa:
Research grants – ActaBiologica, Agios, Astra Zeneca, Bayer, Beigene, Berry Genomics, BMS, Casi, Celgene, Exelixis, Genentech/Roche, Halozyme, Incyte, Mabvax, Puma, QED, Sillajen, Yiviva; consultancy – Agios, Astra Zeneca, Autem, Bayer, Beigene, Berry Genomics, Celgene, CytomX, Debio, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Gilead, Helio, Incyte, Ipsen, Loxo, Merck, MINA, Polaris, QED, Redhill, Silenseed, Sillajen, Sobi, Therabionics, Twoxar, Vector, Yiviva
To view the poster, click here
Back to previous page






